For this simulation, we have provided examples of the three states a system and it's surroundings can be in. For open and closed systems, a boiling pot of water on a hot plate was used to provide a simple yet effective explanation of the difference between the two states. We have also provided an example of an isolated system involving a cooler that has been isolated in space. The images and their explanations can be found below.
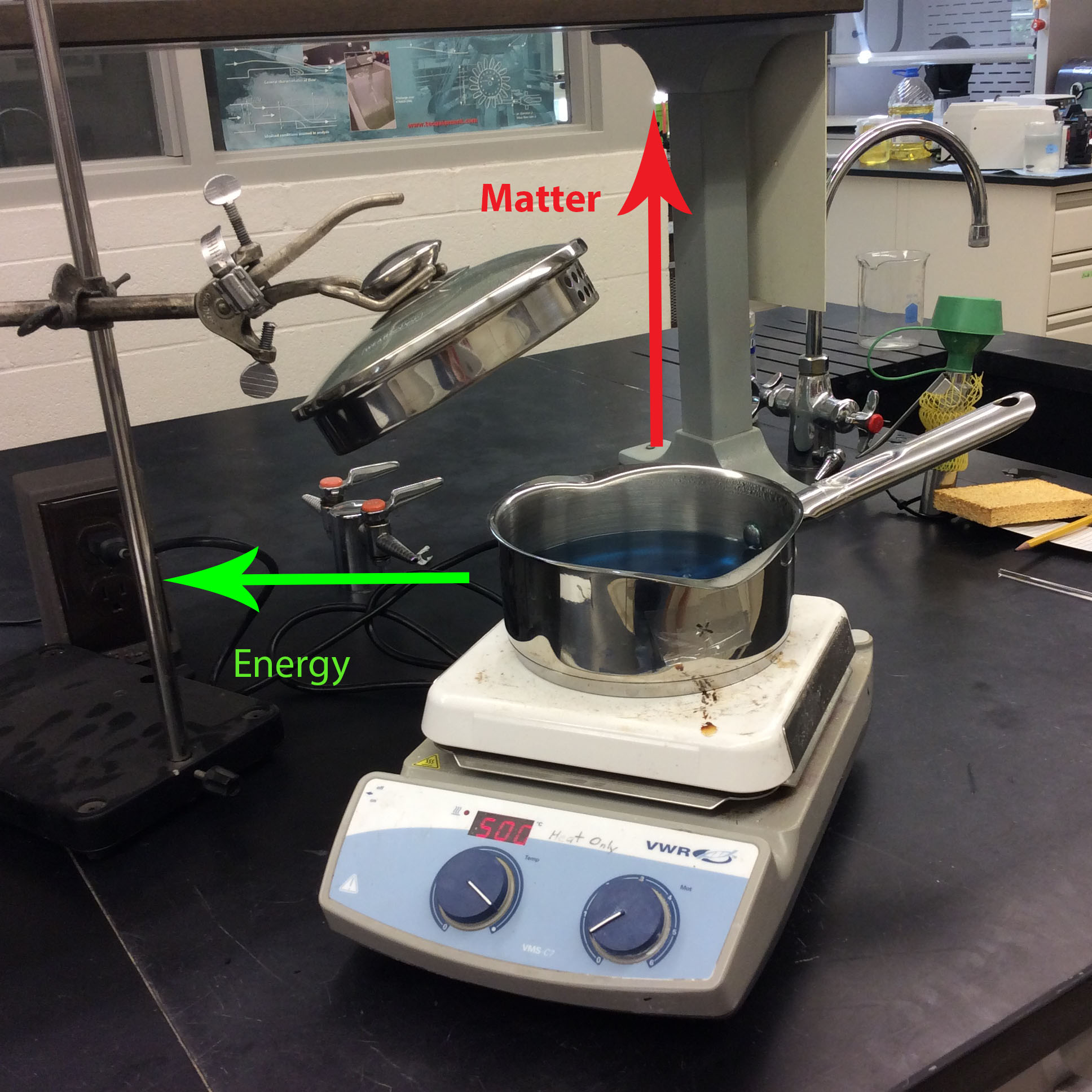
In an open system, both matter and energy can be exchanged with the system's surroundings freely. In this example with the pot of water boiling, matter is leaving the system in the form of steam. Energy is also leaving and entering the system in the form of heat.
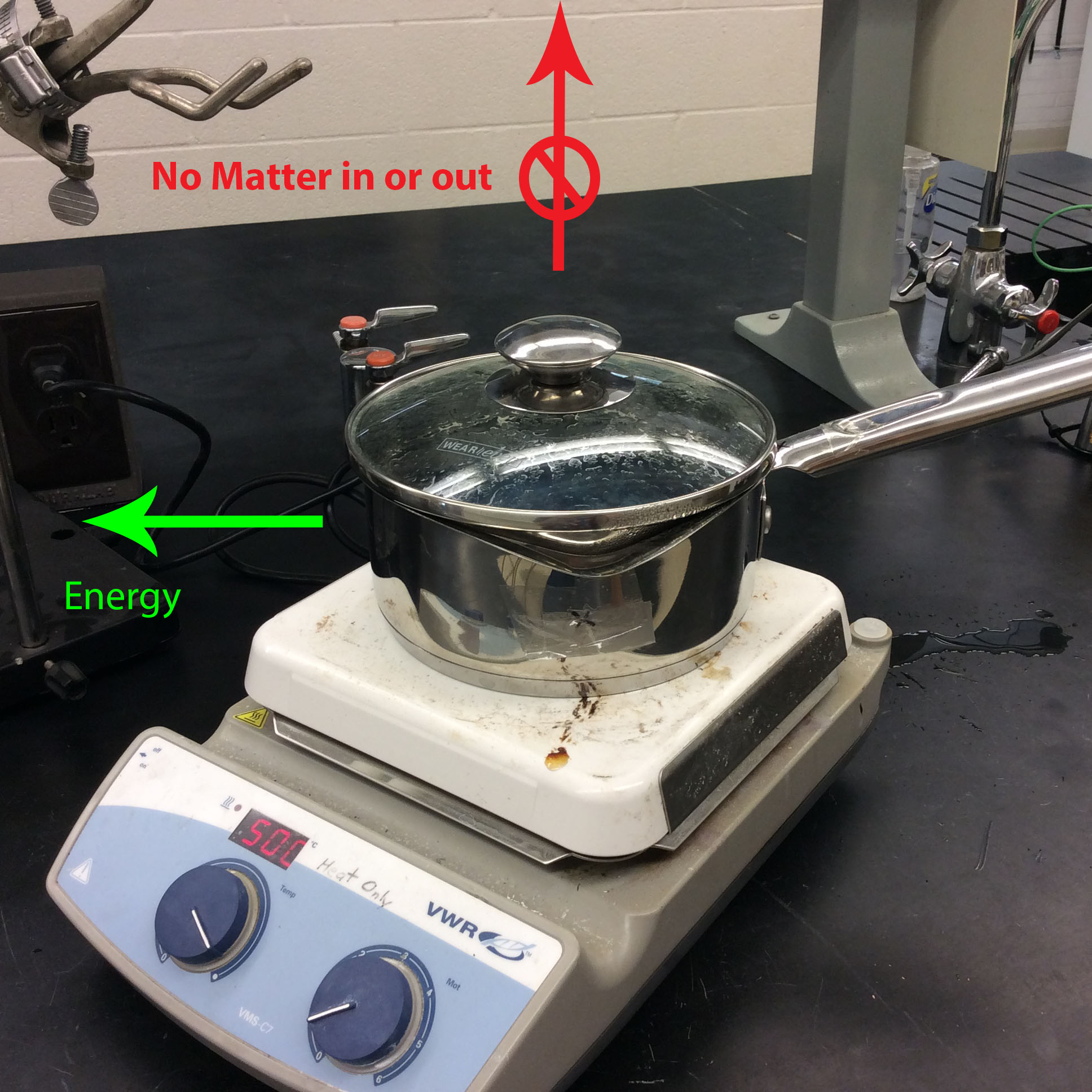
In a closed system, only energy can be exchanged between the system and the surroundings. In the same example of boiling a pot of water, putting the lid on the system changes it from open to closed. This is because when the lid is on the pot, matter can no longer escape. However, energy in the form of heat can still enter and leave the system.

(Background image from: http://earthspacecircle.blogspot.com/p/space-photos-and-wallpapers.html)
In an isolated system, neither matter nor energy can be exchanged between the system and the surroundings. In this example, we have a cooler that has been isolated in space. The cooler does not allow matter or energy to escape or enter. Therefore, the cooler is an example of an isolated system.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Template by OS Templates